
A Revolutionary Breakthrough in Biomedical Testing: Sustainable Amebocyte Production for LAL Testing to Detect Bacterial Contamination
Limulus Amebocyte Lysate (LAL) Can Now Be Produced Without Harvesting the Blood of ~800,000 Horseshoe Crabs each Year to Restore their Natural Population
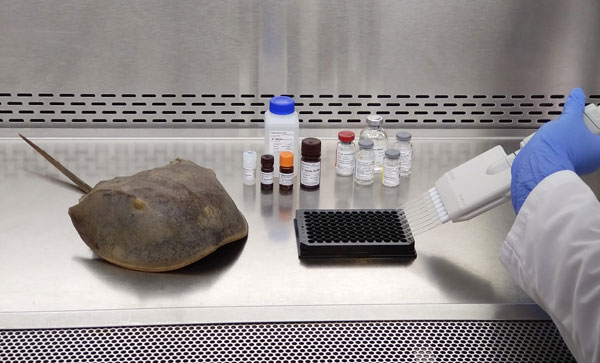
Source: MiBiLab
AmeboGenesis has achieved a significant milestone with the introduction of a sustainable Amebocyte production process. This innovative technology produces bio-identical Amebocytes necessary for the production of LAL for medical endotoxin testing without the need for harvesting horseshoe crab blood. The blood of horseshoe crabs, a source of Limulus Amebocyte Lysate (LAL), is a critical component in ensuring the safety of injectable drugs, vaccines, and implantable devices worldwide. The traditional method of obtaining LAL involves the harvesting and bleeding of ~800,000 horseshoe crabs annually, with a survival rate post-harvest that raises concerns for the Horseshoe Crab’s survival as an endangered species.
Limulus Amebocyte Lysate (LAL)


This pioneering approach by AmeboGenesis, enabling the expansion and differentiation of Amebocyte precursors in cell cultures, marks a first in the world. The resulting cultured cells demonstrate identical genetic, morphological, biochemical, and functional characteristics to their native counterparts. They have been functionally validated to respond to endotoxins, meeting the industry’s gold standard for efficacy. As a leader in biotechnology innovation, AmeboGenesis is dedicated to making a profound impact on the biomedical, pharmaceutical, and food safety sectors.
Horseshoe Crabs. A Prehistoric Creature. A Cruel Process.

Image Source via Business Insider.

Horseshoe Crabs Are an Unsustainable Supply Source & Potentially Endangered Species
All LAL is currently obtained by collecting amebocytes from the blue colored blood of living horseshoe crabs. 20-25% of their blood volume is physically extracted and can result in a significant mortality rate of up to 30%. This is contributing toa serious population decline along with the challenges of climate change and pollution. These potentially endangered prehistoric animals cannot be farmed but must be harvested in the wild.
There is increasing demand for LAL which is not being met by new recombinant endotoxin detection technologies. The market has clearly shown that it demands the continued use of LAL testing.
The AmeboGenesis™ Proprietary Technology Offers a very unique opportunity to:
Leverage the well-established LAL test reputation
Provide a sustainable source of amebocytes
Allow horseshoe crab populations to return to a natural state
Advance the technology, reliability, and reproducibility
Greatly increase LAL supply to expand market to new uses in food, agriculture and water safety testing, etc.
Genetically consistent source of amebocytes, namely one horseshoe crab instead of a combination of amebocytes from countless horseshoe crabs.
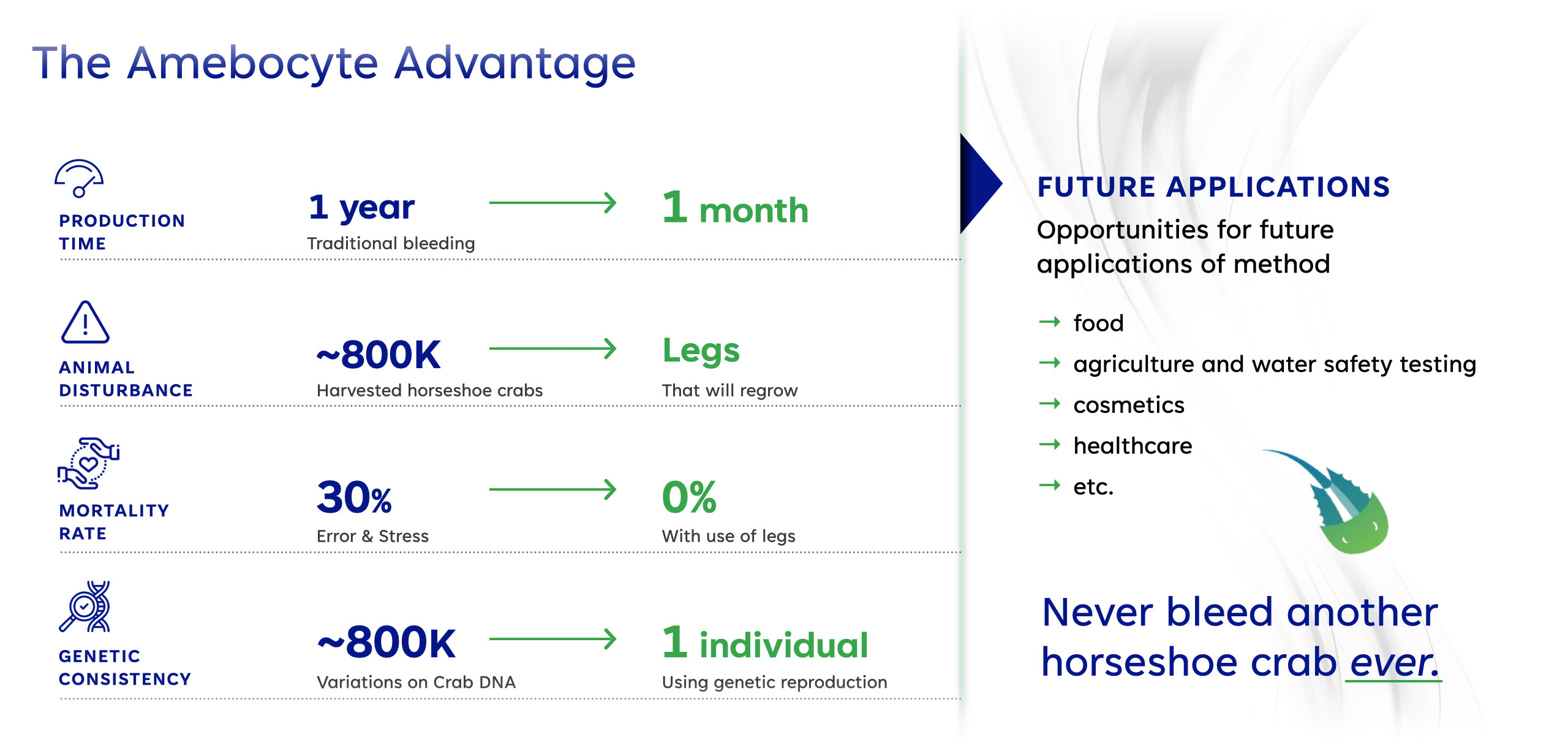
With an Unlimited Supply of LAL, AmeboGenesis Will Address Broad Market Opportunities in Medical, Food and Water Testing, and More…
LAL is currently necessary for the testing of vaccines, insulin, local anesthetics, Botox, filler, dialysis machines, IV infusions, and implantable devices, such as joint replacements and coronary artery stents. Commercial testing for contamination in food and beverages, water treatment and distribution, healthcare settings and environmental monitoring. As consumer confidence in the safety of food, cosmetics and personal care products is diminishing, the need for personal LAL-based test kits is increasing. The current limits for the availability of Amebocytes for LAL production does not allow the expansion of LAL testing to serve these rapidly expanding and essential needs.
Our Commitment to Bioethics
Traditionally, the biomedical industry has relied on Horseshoe Crabs for Amebocytes, vital for pyrogen testing. The commercialization of our revolutionary process for manufacturing Amebocytes represents a significant ethical advancement in the Biomedical field. The AmeboGenesis platform will eliminate the need to bleed Horseshoe Crabs and addresses critical global concerns regarding wildlife conservation and sustainable practices. It will end our dependency on animal sources, directly contributing to the conservation of these ancient creatures, whose populations have been endangered. This aligns with our ethical commitment to biodiversity and environmental stewardship as we advance medical science to no longer need to extract products from animals for medical use.
